May 16, 2020 This means that there are 45 neutrons in the most common, and therefore most stable isotope of bromine. The number of protons is equal to the number of electrons in an electrically neutral atom, so there are 35 electrons in a bromine atom. Secondly, how many bonds can bromine have? Bromine will normally form one covalent bond. No its not 35 that includes valence electrons. The core electrons are those in the filled energy level. Therefore it is C. It has the 1s, 2s, 2p, 3s, 3p, 3d filled making twenty eight core.

Electron Configuration For Bromine
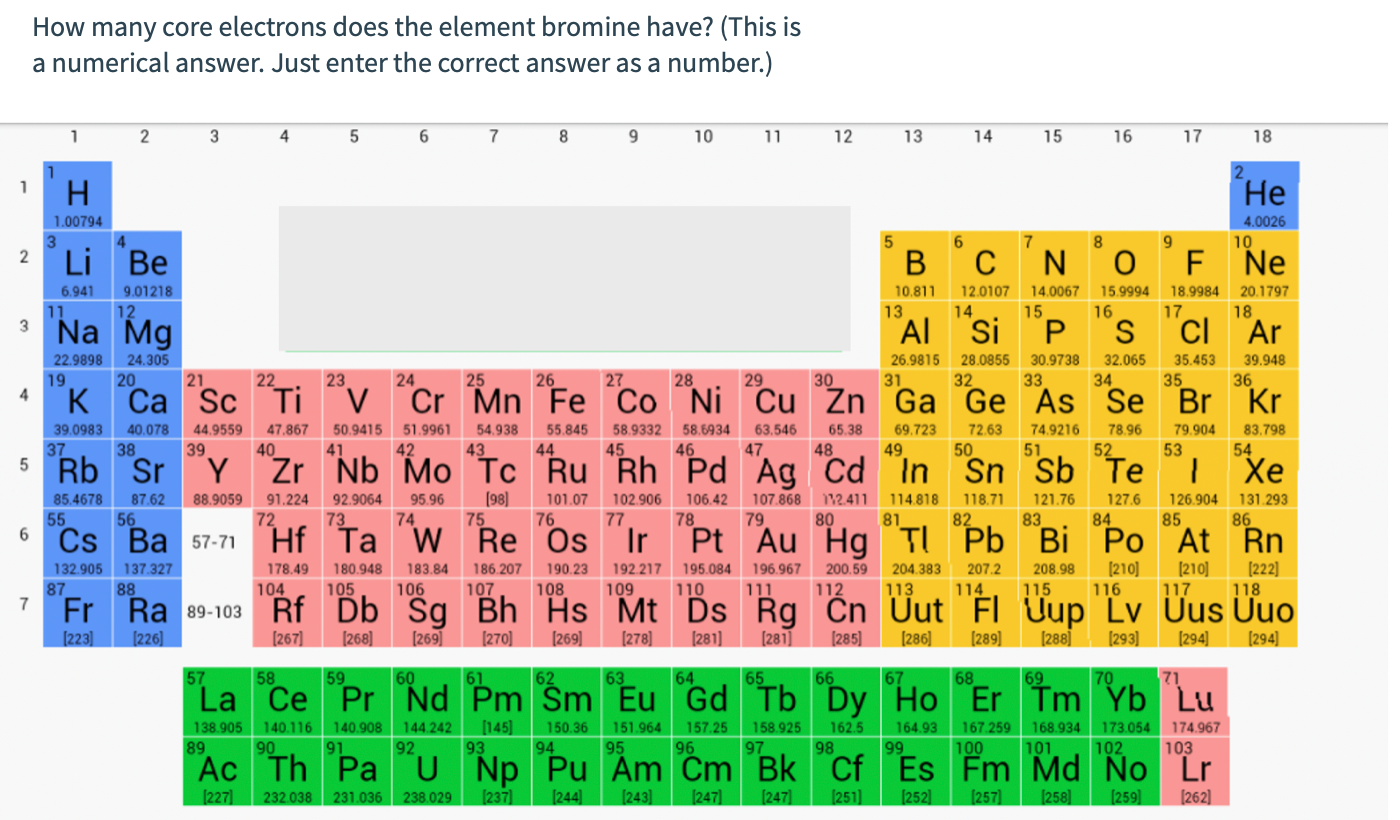
Bromine Orbital Diagram
 PDF
PDF